FDA Approves New Treatment for Macular Degeneration
A new drug offers hope to the one million Americans afflicted by macular degeneration.
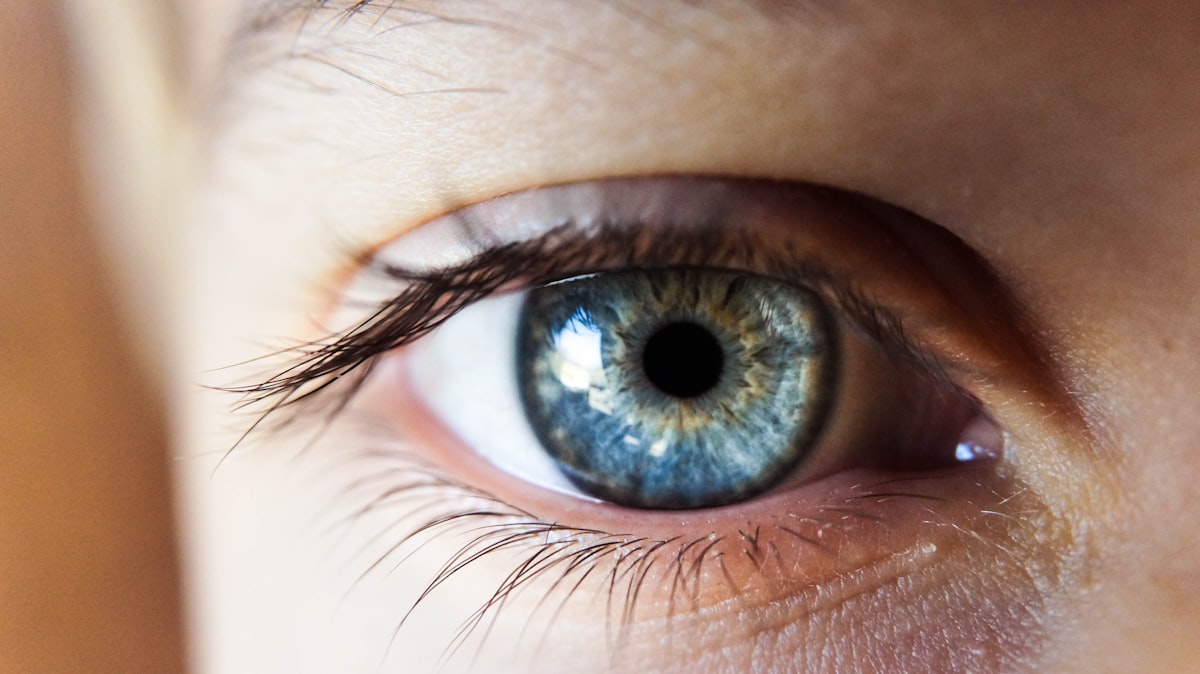
The U.S. Food and Drug Administration has approved a new treatment for macular degeneration, a leading cause of blindness that afflicts more than one million people in the United States, the drug's manufacturer said.
SYFOVRE, developed by Apellis Pharmaceuticals, is given by injection to treat geographic atrophy (GA) secondary to age-related ("dry") macular degeneration (MD).
“The approval of SYFOVRE is the most important event in retinal ophthalmology in more than a decade,” said Eleonora Lad, M.D., Ph.D., lead investigator for the OAKS study, director of ophthalmology clinical research, associate professor of ophthalmology, Duke University Medical Center.
“Until now, there have been no approved therapies to offer people living with GA as their vision relentlessly declined. With SYFOVRE, we finally have a safe and effective GA treatment for this devastating disease, with increasing effects over time,” Lad said.
Syfovre tested in trials
The approval of SYFOVRE is based on positive results from the Phase 3 OAKS and DERBY studies at 24 months across a broad and representative population of patients.
In the OAKS and DERBY studies, SYFOVRE reduced the rate of GA lesion growth compared to sham and demonstrated increasing treatment effects over time, with the greatest benefit (up to 36% reduction in lesion growth with monthly treatment in DERBY) occurring between months 18-24.
“For the first time ever, we are celebrating the approval of a treatment for GA,” said Jeff Todd, president and chief executive officer, Prevent Blindness. “This is a historic and hopeful day for all GA patients and their care partners, who have been waiting for a treatment for this relentless form of vision loss.”
GA is an advanced form of AMD. It is a progressive and irreversible disease caused by the growth of lesions, which destroy the retinal cells responsible for vision. The vision loss caused by GA severely impairs independence and quality of life by making it difficult to participate in daily activities. On average, it takes only 2.5 years for GA lesions to start impacting the fovea, which is responsible for central vision.
Apellis is offfering ApellisAssist, a program designed to help SYFOVRE patients by providing a system inclusive of insurance support, financial assistance for eligible patients, disease education, and ongoing product support. Patients and healthcare providers can call 1-888-273-5547 for more information.
SYFOVRE is expected to be available by the beginning of March through specialty distributors and specialty pharmacies nationwide. A marketing authorization application for SYFOVRE is under review by the European Medicines Agency with a decision expected in early 2024. In addition, a marketing application has been submitted to Health Canada.
