Cheap Oximeters Removed from Online Sites after Accuracy Questioned
Amazon and eBay are among companies that removed a number of blood oxygen testing devices from their sites after the British consumer group Which? raised questions about whether the devices could be legally sold in the UK.
The consumer champion found 11 out of 15 cheap pulse oximeters bought from online marketplaces failed to comply with UK and EU law when it assessed them. The survey didn’t include North American sites and there is no indication a similar problem exists in the U.S.
“It is very concerning that our investigation found these medical devices for sale without the required safety markings or brazenly claiming to be approved by the NHS – and the biggest online marketplaces were not picking up on these red flags,” said Natalie Hitchins, Which? Head of Home Products and Services.
“Consumers should be wary of cheap oximeters sold on online marketplaces.”
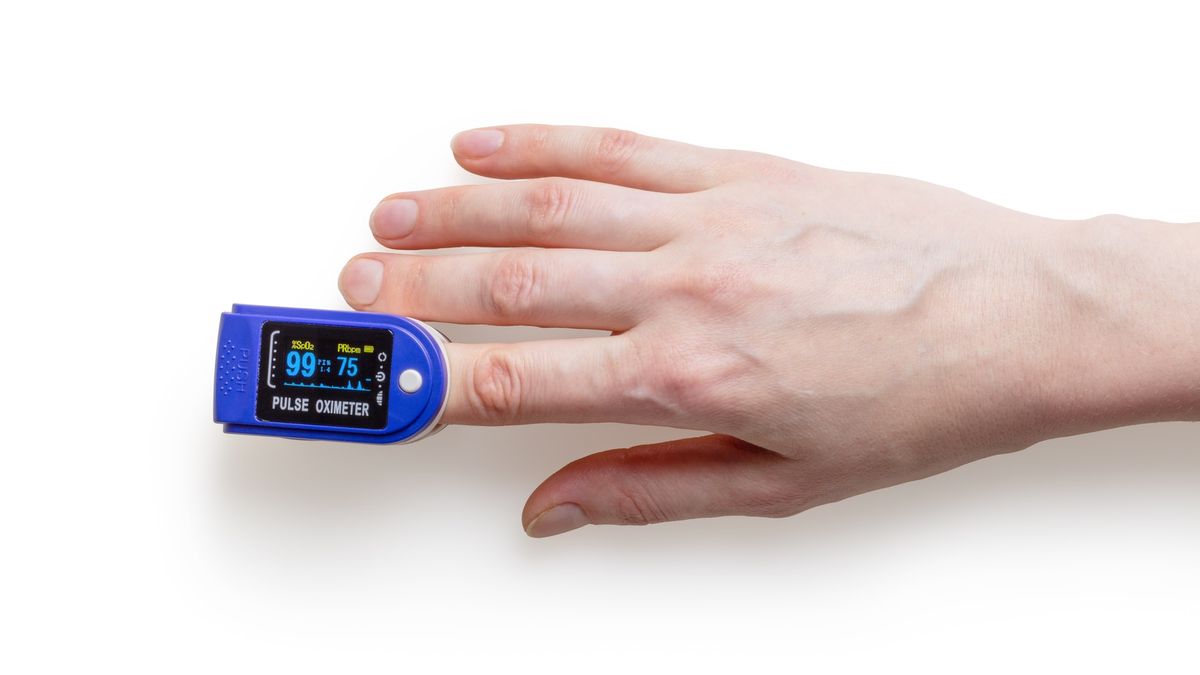
Pulse oximeters have become increasingly popular since the Covid-19 pandemic began. They measure oxygen levels in the blood which can drop to dangerously low levels without the patient noticing, in what is known as “silent hypoxia”.
Pulse oximeters are being used to assist vulnerable patients with home monitoring of Covid or post-Covid symptoms. Patients may be given a pulse oximeter by their personal physician but many consumers also order the devices on their own.

Endorsement labels questioned
The products that Which? analyzed featured prominently in search listings, had lots of reviews and in some cases featured marketplace endorsement labels.
Which? found that one product being sold via the online marketplaces was missing its Conformitè Europëenne (CE) Mark (the EU’s conformity marking) completely, while others had CE marks that failed to comply with the regulations governing medical devices.
Some falsely claimed to be approved by the UK’s National Health Service (NHS) or used the NHS wording or logo to look more legitimate, Which? said. This could potentially be a breach of consumer protection law.
A UK Department of Health and Social Care spokesperson told Which?: “The NHS does not approve or endorse any medical devices, including oximeters.”
When Which? notified the marketplaces about the incorrect certification on the products it found, they took them down – although five devices had already disappeared from sale before the consumer champion contacted the marketplaces.
All of the pulse oximeters Which? tested passed its accuracy tests without any major issues, so they were able to measure the blood oxygen saturation of each of Which?’s panel with an acceptable error margin.
However, Which? said buying from an unknown seller could come with risks, as the high number of uncertified models shows. It can be a lottery for consumers to know what they are getting and other snapshot tests Which? has done, such as digital thermometers, have uncovered products that can give inaccurate readings.
